Denise gives a seminar on the work in two new papers
Dai, Guo et al, Science, in press
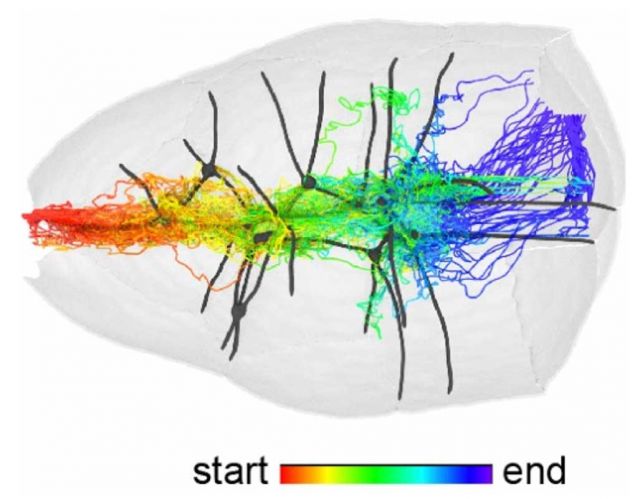
Cell motility is essential for embryonic development, adult homeostasis, tumor cell dissemination, and immunity. Migrating cells navigate physical features of their microenvironment, however the in vivo significance of tissue topography for pathfinding is mostly unknown. Dai, Guo et al. use Drosophila border cells to study path selection in vivo. Experiments, mathematical modeling and simulations show that tissue microtopography provides an energetically-favorable path of least resistance while chemoattractants supply orthogonal guidance information, and cell-cell adhesion contributes traction. The results provide insight into how cells integrate and prioritize topographical, adhesive, and chemoattractant cues to choose one path amongst many.
Miao et al., Dev Cell, 2020
During embryonic development and cancer metastasis, migratory cells must establish stable connections with new partners at their destinations. Here we establish the Drosophila border cells as a model for this multistep process. During oogenesis, border cells delaminate from the follicular epithelium and migrate. When they reach their target, the oocyte, they undergo a stereotypical series of steps to adhere to it, then connect with another migrating epithelium. We identify gap-junction-forming Innexin proteins as critical. Surprisingly, the channel function is dispensable. Instead, Innexins 2 and 3 function within the border cells, and Innexin 4 functions within the germline, to regulate microtubules. The microtubule-dependent border cell-oocyte interaction is essential to brace the cells against external morphogenetic forces. Thus, we establish an experimental model and use genetic, thermogenetic, and live imaging approaches to uncover the contributions of Innexins and microtubules to a cell biological process important in development and cancer.